The hunter-gatherer groups at the heart of a microbiome gold rush
Scientists have been looking for health-promoting microbes in the feces of people from traditional communities—some of whom feel exploited.

We’re all teeming with microbes. We’ve got guts full of them, and they’re crawling all over our skin. These tiny, ancient life forms have evolved with us. And over the last couple of decades, scientists have come to realize just how important they are to our health and well-being. They help extract nutrients from our food, influence the way our immune systems work, and can even send signals to our brains that play a role in our mental health.
But some researchers believe our microbiomes are in crisis—casualties of an increasingly sanitized, industrialized, and antimicrobial way of life. Disturbances in the collections of microbes we host have been associated with a whole host of diseases, ranging from arthritis to Alzheimer’s.
“It’s very clear in industrialized nations we have lost many species that were probably fundamental to human evolution,” says Justin Sonnenburg, a microbiome scientist at Stanford University. “They’ve just become extinct.” Some have seemingly disappeared before we’ve even had a chance to figure out what they do.
Some might not be completely gone, though. Scientists believe many might still be hiding inside the intestines of people who don’t live in the polluted, processed, and antimicrobial-laden environment that most of the rest of us share. They’ve been studying the feces of people from hunter-gatherer societies like the Yanomami, an Indigenous group in the Amazon, who appear to still have some of the microbes that other people have lost.

And so the race is on to find those missing microbes. Both academics and companies are building catalogues of microbes seen in hunter-gatherer societies, and attempting to re-create this microbial brew as a treatment for people in industrialized societies. The hope is that with the proper mix of microbes, many people might gain protection from disorders, like depression and metabolic disease, that seem to affect people living in industrialized societies at much higher rates. But there is a rather major catch: we don’t know whether those in hunter-gatherer societies really do have “healthier” microbiomes—and if they do, whether the benefits could be shared with others.
At the same time, members of the communities being studied say some projects aren’t being done ethically or equitably. Even recent research projects have taken biological samples without consent and attempted to artificially manipulate the way hunter-gatherers eat and live, says Shani Mangola, a member of the much-studied Hadza society in Tanzania. He and others are concerned about the risk of what’s called biopiracy—taking natural resources from poorer countries for the benefit of wealthier ones.
“Some people don’t understand [and ask], Why are these people taking my hair? Why are they taking my poop?” says Mangola. “They need to understand what the research is about, what the impact is, and what value it brings to the communities and to the world.”
Microbes as medicine
The idea of repopulating our guts with stuff from healthy people goes back a long way. The first known documentation of the idea of fecal transplants dates back to fourth-century China. At that time, a fecal slurry known as “yellow soup” was served up as a broth to people with food poisoning and diarrhea.
Humans might not have had as sophisticated an understanding of gut microbiota back then, but the goal was the same as it is now—to share health. Because our gut microbes are shed in our feces, you can use poo from a healthy person to repopulate the gut of someone who’s sick. These days, fecal transplants are routinely performed for people with stubborn Clostridium difficile infections. The procedure, which relies on donations from healthy volunteers, can work remarkably well, with a success rate over 80%.
Scientists have been exploring the use of fecal transplants for other diseases too. Microbes in your gut can influence the way your digestion works, and how your intestines function in general. But the microbes also produce a range of chemicals that influence other aspects of our health, including inflammation and brain function. Clinical trials are underway for the use of fecal transplants in anorexia, diabetes, Parkinson’s disease, and Crohn’s, among others. And they are also being investigated for their potential to enhance the way people respond to other treatments.
But microbial transplants can be messy. While hospitals will always filter donations and check for some particularly nasty bugs, we can never be completely sure a fecal transplant won’t harbor bacteria that cause disease in a recipient even if they are totally innocuous in a donor’s gut. And because these are complex mixtures of microbes, doctors won’t usually know exactly what they’re squirting down a person’s throat or up their anus.
The goal is to have a defined mix of known microbes. Not only should we know what the bugs are, but we should know what they do—what they feed on and what they produce. We should know that they are likely to benefit a person before we deliver them.
Researchers interested in creating these sorts of microbial cocktails are looking beyond what we tend to understand as “healthy” when it comes to donors. Yes, we should be looking for microbes in the feces of people who don’t have infections or diseases, but that’s not enough. Chronic diseases linked to inflammation have taken off just as we’ve begun to live more sanitized lives. And if a lack of protective microbes is to blame, even the healthiest people in industrialized groups aren’t going to provide much help.
Instead, scientists want to cast the net wider: to find microbes that evolved with us, but that many of us have since killed off or lost. The missing microbes.
Missing microbes
Those of us who live in industrialized societies have changed the habitats of our gut microbes to a fairly drastic degree. We use antibiotics and antibacterials and eat a diet of novel ingredients and heavily processed foods.
As a result, microbiologists believe, we’ve been killing off some of the microbes that humans once carried. Compare modern-day fecal samples with ancient ones, and there are clear differences. The microbiomes of today are less diverse, with more of some bugs and fewer of others. Scientists believe that some of the ones that are missing have very important functions, like breaking down certain carbohydrates and producing chemicals that might be important for gut health.
Some people call it a great extinction. And the decline in these microbes has been linked to an uptick in a range of chronic diseases like asthma, diabetes, and inflammatory bowel disease.
Aleksandar Kostic, a microbiologist at Harvard Medical School, wants to know what microbes our ancestors did have. A couple of years ago, he and his colleagues looked for microbial DNA in eight samples of ancient human feces collected from the southwestern United States and Mexico. These remains, known as paleofeces, were estimated to be between 1,000 and 2,000 years old.
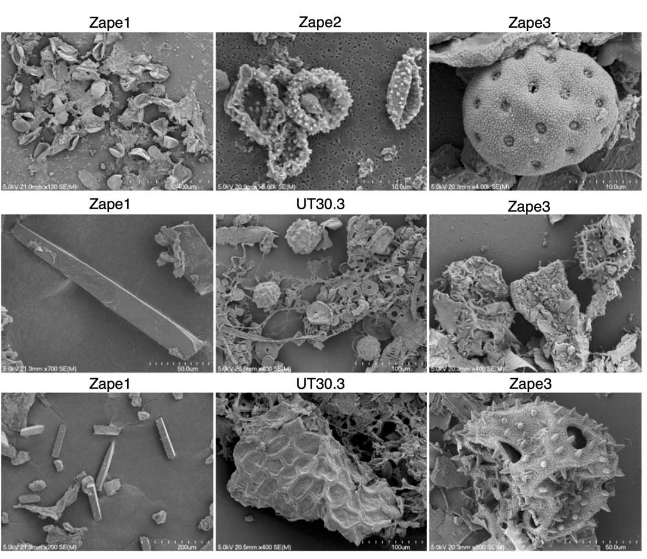
When Kostic and his colleagues compared the fossilized poo with modern-day microbiome samples from people from eight different countries, they found significant differences. But some samples were more similar than others.
Specifically, modern-day samples from people who live in “nonindustrialized” communities had a lot more in common with the ancient feces. “The paleofeces and the Yanomami samples almost matched,” says Emma Allen-Vercoe, a microbiologist at the University of Guelph in Canada, who was not an author of the study.
In their write-up, Kostic and his colleagues conclude that “similar future studies tapping into the richness of paleofeces will not only expand our knowledge of the human microbiome, but may also lead to the development of approaches to restore present-day microbiomes to their ancestral state.”
Paleofeces are hard to come by, though. So microbiologists have turned to people in those “nonindustrialized” communities instead. “There’s a lot of value in studying modern hunter-gatherers,” says Kostic. “We were all hunter-gatherers for the vast majority of human history.”
Kostic thinks that a more “ancient” microbiome might help protect such people from some of the chronic diseases that plague industrialized groups. He points to research suggesting that Indigenous groups that rely on hunting, gathering, fishing, and farming appear to have a much lower risk of disorders like coronary artery disease, for example.
The idea is that such groups have maintained a microbiome that hasn’t been subject to the same great extinction that happened among people in industrialized places. Could they be better off because of it?
Perfect poo
While we still don’t know exactly what a perfect microbiome should look like, researchers agree that some microbes are especially important. Just like any ecosystem, our guts are probably home to keystone species—organisms that have an outsize impact on the system as a whole. “Fostering those seems to be good,” says Allen-Vercoe.
Harboring a diverse collection of microbes also seems to be important. People who eat healthy diets and have fewer health complaints tend to have a better mix of microbial species in their guts. The theory is that with a broader range of microbes, a person can benefit from more microbial functions—and the production of more health-promoting chemicals. “The more diversity you have, the more [microbial] genes you carry,” says Allen-Vercoe. “And the more genes you carry, the more biochemical work you can do.”
There is evidence to suggest that people who live in less industrialized environments host a richer diversity of gut microbes. And as industrialization takes shape in a community, its members start to lose this diversity. But it’s still not clear what sort of impact this has on human health. “The more urban, the less diversity,” says Maria Gloria Dominguez-Bello, a microbial ecologist at Rutgers. “What we still don’t know is: What functions are we losing?”
The first step to finding out is to catalogue what microbes we might have lost. To get as close to ancient microbiomes as possible, microbiologists have begun studying multiple Indigenous groups. Two have received the most attention: the Yanomami of the Amazon rainforest and the Hadza, in northern Tanzania.
Researchers have made some startling discoveries already. A study by Sonnenburg and his colleagues, published in July, found that the gut microbiomes of the Hadza appear to include bugs that aren’t seen elsewhere—around 20% of the microbe genomes identified had not been recorded in a global catalogue of over 200,000 such genomes. The researchers found 8.4 million protein families in the guts of the 167 Hadza people they studied. Over half of them had not previously been identified in the human gut.
Plenty of other studies published in the last decade or so have helped build a picture of how the diets and lifestyles of hunter-gatherer societies influence the microbiome, and scientists have speculated on what this means for those living in more industrialized societies. But these revelations have come at a price.
A changing way of life
The Hadza people hunt wild animals and forage for fruit and honey. “We still live the ancient way of life, with arrows and old knives,” says Mangola, who works with the Olanakwe Community Fund to support education and economic projects for the Hadza. Hunters seek out food in the bush, which might include baboons, vervet monkeys, guinea fowl, kudu, porcupines, or dik-dik. Gatherers collect fruits, vegetables, and honey.
Mangola, who has met with multiple scientists over the years and participated in many research projects, has witnessed firsthand the impact of such research on his community. Much of it has been positive. But not all researchers act thoughtfully and ethically, he says, and some have exploited or harmed the community.
One enduring problem, says Mangola, is that scientists have tended to come and study the Hadza without properly explaining their research or their results. They arrive from Europe or the US, accompanied by guides, and collect feces, blood, hair, and other biological samples. Often, the people giving up these samples don’t know what they will be used for, says Mangola. Scientists get their results and publish them without returning to share them. “You tell the world [what you’ve discovered]—why can’t you come back to Tanzania to tell the Hadza?” asks Mangola. “It would bring meaning and excitement to the community,” he says.
Some scientists have talked about the Hadza as if they were living fossils, says Alyssa Crittenden, a nutritional anthropologist and biologist at the University of Nevada in Las Vegas, who has been studying and working with the Hadza for the last two decades.
The Hadza have been described as being “locked in time,” she adds, but characterizations like that don’t reflect reality. She has made many trips to Tanzania and seen for herself how life has changed. Tourists flock to the region. Roads have been built. Charities have helped the Hadza secure land rights. Mangola went abroad for his education: he has a law degree and a master’s from the Indigenous Peoples Law and Policy program at the University of Arizona.

At the same time, land-grabbing and business expansion are limiting the natural resources available to the Hadza. Forty years ago, Mangola says, animals were relatively easy to find in the bush, and his community ate meat almost every day. Nowadays, hunters have to travel much farther to find animals. Communities might eat meat once a week. The Hadza people increasingly use money from tourists to buy food from nearby farms and villages, he says.
This reality doesn’t fit with the narrative of an existence that has barely changed since ancient times. Mangola says he has seen scientists try to encourage Hadza people to adopt more traditional diets and lifestyles for research projects. “Some of them come here and stop the Hadza eating their normal food—they ask them to eat as if they were eating years ago,” he says. “[They should] tell the world the truth: there’s not enough food in the bush. People are going borrowing and begging for food because the bush is gone.”
Several people pointed to work done by Jeff Leach, an archaeology writer who also studied microbiomes in the Hadza people. Leach infamously used a turkey baster to give himself a fecal transplant from a member of the Hadza community in an effort to improve his own gut health. (None of the people contacted by MIT Technology Review know the full details of the impact this stunt had on Leach’s health.)
“Taking advantage of an Indigenous population and using their microbes to try to reinstate health in somebody from a wealthy, industrialized nation, I think, is a problematic thing to do,” says Sonnenburg. He doesn’t think such experiments should never be done—just that the ethical implications should be thoroughly explored, and that the Hadza should be fully informed and consent to the research.
“The Hadza were so sad about that,” says Mangola. “He used to be my friend.” MIT Technology Review attempted to contact Leach via email but did not receive a response.
A fair share
An ocean away, David Good is trying to forge a different path forward for microbiome research. Good had an unusual childhood. Half American and half Yanomami, he split the first five years of his life between the Amazon rainforest in Venezuela and suburban New Jersey.
Good’s father, an American anthropologist, had met his mother, then a member of the Yanomami Hasapuwe-teri community, while studying aspects of the group’s diet, Good says, adding: “He completely fell in love with this world.”
But when his mother moved to New Jersey, she struggled to adapt. “We were just trying to live this intercultural, international lifestyle, with one foot in the jungle and one foot in the suburbs,” he says.
Ultimately, Good stayed in the US with his father and siblings, while his mother moved back to be with the Yanomami. “That would be the last time I would see her for 20 years,” he says.

In 2011, Good, who is now a microbiome researcher and PhD candidate at the University of Guelph, made the challenging and dangerous journey back to find his mother.
His visits since have been about more than reestablishing family ties.
On visits to his mother’s Yanomami community, he noticed an absence of chronic diseases—including mental-health disorders. “You don’t see depression; you don’t see PTSD,” he says. “The concept of suicide is just unfathomable to them.”
He thinks that the Yanomami’s microbiomes might be benefiting their health, probably via their diet, which is free of sanitized and factory-processed foods. “The Yanomami don’t have the choice to eat bad food,” says Good. “Everything that they eat—whether it’s a monkey, capybara, or plantains—is all going to be good for their microbiome.”
Good supports the search for missing microbes in the guts of the Yanomami, but he stresses that any research should involve an exchange with the community. “[Scientists] can’t just parachute down and extract, and then leave,” he says. “Indigenous peoples are co-producers of knowledge, and their microbiome is not just sort of this passive thing that is there for us to take and leave.”
The Yanomami have had experiences similar to those of the Hadza. “They’re angry that scientists have come, taken their samples, and never come back,” says Good. The results of the research aren’t shared with them. And neither are any potential profits.
He is working to redress that with the Yanomami Foundation, a nonprofit organization that aims to support and steward ethical research with the Yanomami by seeking consent and addressing the wishes and needs of the community.
“The microbes belong to David, essentially, and the Yanomami Foundation he’s started. Essentially we’re borrowing this stuff,” says Allen-Vercoe. “And the idea is that if we find something interesting that has some [intellectual property] … that will go to benefiting the Yanomami.”

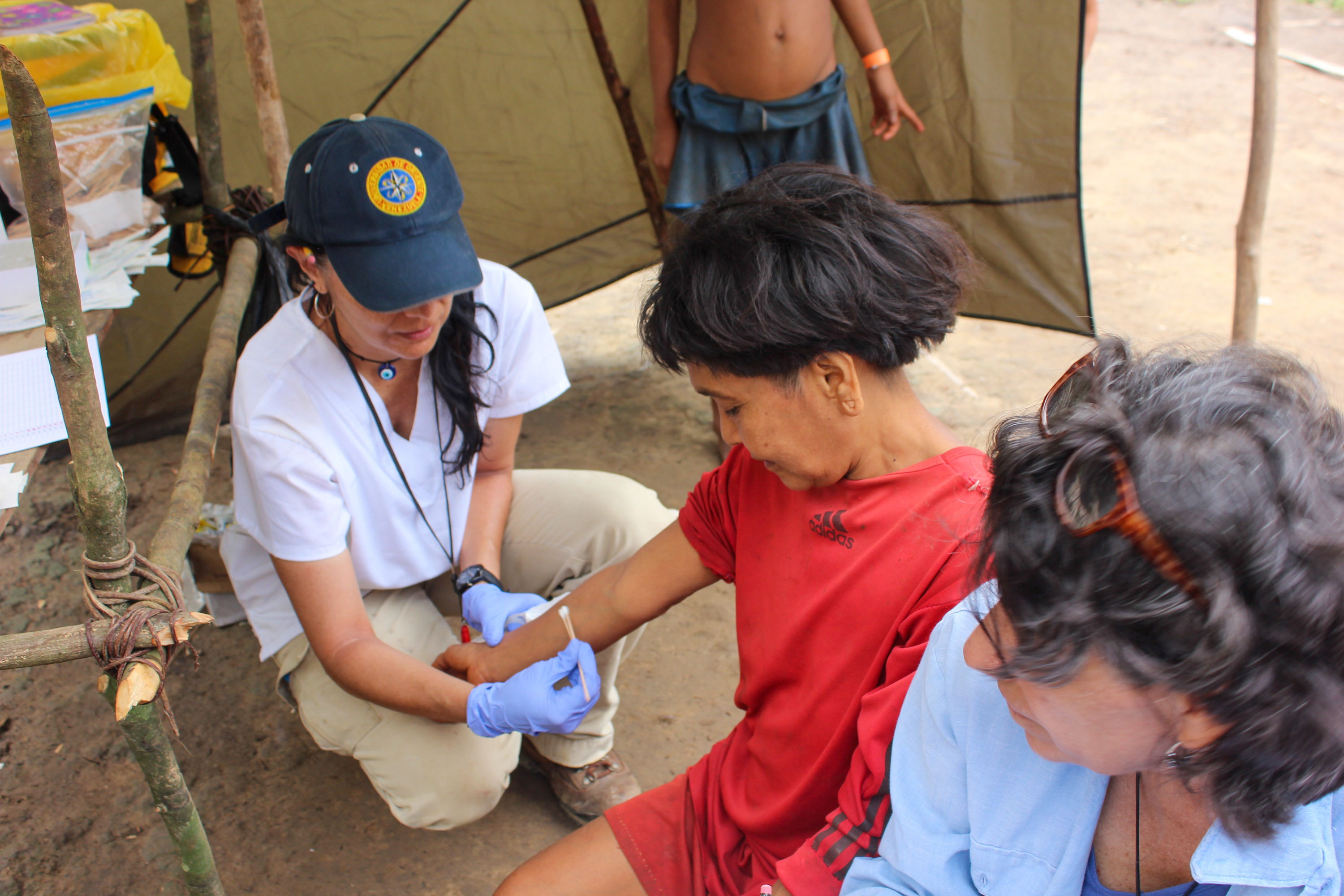
Researchers collect samples from consenting villagers, including Yarima (above), with the understanding that the results and benefits will be shared with the Yanomami people.
The Yanomami want more resources for health care to help tackle the spread of diseases like malaria and tuberculosis. They want technology that allows them to follow news and communicate. And they want bilingual education, says Good. “The Yanomami people are not just, like, these passive animals in a zoo just to be observed,” he says. “They’re human beings; they want to engage with the outside world, and they want innovation.”
Larry Weiss, CEO of Symbiome, a company that sells cosmetic skin-care products, has been working with Good to study the skin microbiome of the Yanomami, which he describes as being “biologically intact.” “Things like acne, eczema, rosacea, and psoriasis don’t exist anywhere in hunter-gatherer populations,” Weiss says. He hopes that by learning more about the skin microbes of the Yanomami, he’ll be able to “restore health” in other populations.
Symbiome has paid for the genome sequencing of samples Good has collected from the Yanomami, says Weiss. The company has also “sustainably harvested” plants from an environment in Brazil similar to the one in which the Yanomami live. It sells a product made by adding microbes—some of which are found on the skin of Yanomami people—to fermented, ground-up rainforest plants. “A proportion of the profits go to supporting the Yanomami Foundation,” says Weiss. He won’t say what that proportion is.
Sharing the benefits
All of those MIT Technology Review spoke with agree that scientists need to make an effort to share their results with the communities they study. Sonnenburg hasn’t been to Tanzania himself, but he has sent infographics to help a colleague attempt to explain his research findings to the Hadza participants.
Everyone interviewed also wants to avoid engaging in biopiracy. “It’s the idea of industrialized nations going into low- and middle-income countries and taking advantage of their resources for their own gain,” says Sonnenburg. “Going into these areas that are under-resourced and taking from them, and not giving them ownership over these things that are coming from their people and their lands.”



Naturalist and translator Douglas Symbeye shows members of the Hazda an infographic, to help explain the nature of the research.
“We are looking to extract species to better our own health without any return to the community,” says Crittenden. “If that’s not biopiracy, I don’t know what is.” Crittenden also uses the term “scientific colonialism.” “[It] often happens when elite groups—such as white American researchers—take resources from less influential communities,” she says.
“I’m the first to admit that I think I made a lot of mistakes, and I did things incorrectly,” adds Crittenden, who has worked with the Hadza since 2004.
Back in the early 2010s, she was performing cutting-edge research on the microbiomes of Hadza people. But an interview she conducted in 2013, with a woman who was holding her granddaughter under a tree, gave her pause. “She shared with me that she no longer wanted to participate in any work that required biological samples—saliva, breast milk, urine, blood, or feces,” says Crittenden. “She said that she was exhausted with all of the research teams that come in, do a project… don’t speak Swahili, don’t know the community … she was getting tired of giving parts of her body to strangers.”
Since then, Crittenden has changed the way she performs this research. “I now do exclusively community-based and community-inclusive work,” she says. Some of her recent work has focused on how Hadza children avoid harm, and on differences in emotion processing between the Hadza and people in the US. She no longer collects or studies biological samples, either.
“It’s important that we talk about the mistakes that we make, and that we are accountable for the ways in which we may misstep, albeit unintentionally,” she says. “We need to talk about the impact of our work, and not just our intention.”
Scientists who wish to perform research with the Hadza can apply for permits from the Tanzanian government. These permits explicitly state that researchers are not allowed to use their findings for commercial gain, says Sonnenburg.
But even if scientists themselves don’t use that knowledge to create a commercial product, there’s no guarantee that other companies won’t. “Even if you don’t engage personally in the commercial aspect of it, I still think you’re implicated in it,” says Good. “I feel that if we learn about the Yanomami gut microbiome, we publish on it, and then the private industry is going to learn from those publications how to remodel their products … that needs to be shared back to the Yanomami people.”
Mangola thinks that’s “a great idea,” and he hopes something similar can be put in place for the Hadza.
The best microbiome?
Even if these ethical problems are ever solved, scientific ones will endure. For a start, while we believe that microbial diversity is important, we haven’t firmly established anything other than a correlation between health and a more diverse microbiome. Is this diversity responsible for a lack of chronic disease, or is it a consequence of a particular diet that might not even benefit everyone in the same way?
We know that antibiotics can disrupt our gut health. But the details of the link between microbial diversity and health are still largely a mystery. Even if you assume diversity is good, it’s not clear how much is needed—or what’s the best way to foster it.
Not too long ago, the line of thinking was that the more diverse your diet, the better. Now Allen-Vercoe isn’t so sure. People who live in big cities have such a wide range of food options that they can eat a different meal every day of the month. But they are thought to have some of the “least healthy” microbiomes, she says.
And for all we know, people in industrialized societies may have lost microbes because they no longer serve any purpose in our diet. Maybe they would be likely to cause an infection. Maybe doing away with some of them is really no great loss after all. Maybe the rise of chronic illness is only correlated with the loss of microbial diversity, and other factors are responsible.
Because microbes evolve and adapt to their environments, we should expect the microbiomes of people who live in cities to look different from those of people who live in forests. “There is no archetypal microbiome that everyone should [aspire to],” says Good. “Your microbiome is a reflection of your intimate interaction with your surrounding ecosystem, including the foliage, the air, the water, the food—and that all plays a role in driving the diversity of the microbiome within your gut and on your skin.”

Good says his friends have asked him for samples to give themselves transplants. “I’m like, are you kidding?” he says. “You can’t just wholesale transfer the microbiome of the Yanomami to a non-Yanomami and think you’re going to do well.”
Kostic doesn’t think it’s a good idea either, despite the concluding note in his paper. “I think there are dangers to it,” he says. “We don’t quite know how industrialized folks will respond to all of these microbes that we haven’t really seen … in our lifetimes.”
At any rate, hunter-gatherer societies are not definitive paragons of health. While people in these groups may have lower rates of chronic diseases than people in industrialized places, they experience higher rates of infectious diseases. And because of a lack of health care, those diseases are more likely to be fatal. “Hunter-gatherer communities have the highest rates of infant mortality,” adds Crittenden.
At the moment, the hunt for pristine microbiomes is a bit like a gold rush—one in which no one is sure what gold is, exactly, or whether it will ultimately prove valuable. Allen-Vercoe has spent years growing gut microbes in her lab to learn more about the chemicals they produce and how they might affect our health. She and her colleagues study collections of microbes in a bioreactor they call the Robogut, which is designed to simulate the conditions of human intestines.
For all that work, she says, we have got a long way to go before we can start piecing together what a healthy microbiome looks like. “We still don’t know what a perfect microbiome is,” Allen-Vercoe says. Perhaps there is no such thing.
Deep Dive
Biotechnology and health
The Biggest Questions: What is death?
New neuroscience is challenging our understanding of the dying process—bringing opportunities for the living.
I received the new gene-editing drug for sickle-cell disease. It changed my life.
As a patient enrolled in a clinical trial for Vertex’s new treatment, I was among the first to experience CRISPR’s transformative effects.
The lucky break behind the first CRISPR treatment
Gene editing for sickle-cell is here. This is how researchers knew what DNA to change.
Medical microrobots that can travel inside your body are (still) on their way
Microrobots released into the body could bust up clots, deliver cancer drugs, and even guide listless sperm to their target.
Stay connected
Get the latest updates from
MIT Technology Review
Discover special offers, top stories, upcoming events, and more.