The gene-edited pig heart given to a dying patient was infected with a pig virus
The first transplant of a genetically-modified pig heart into a human may have ended prematurely because of a well-known—and avoidable—risk.
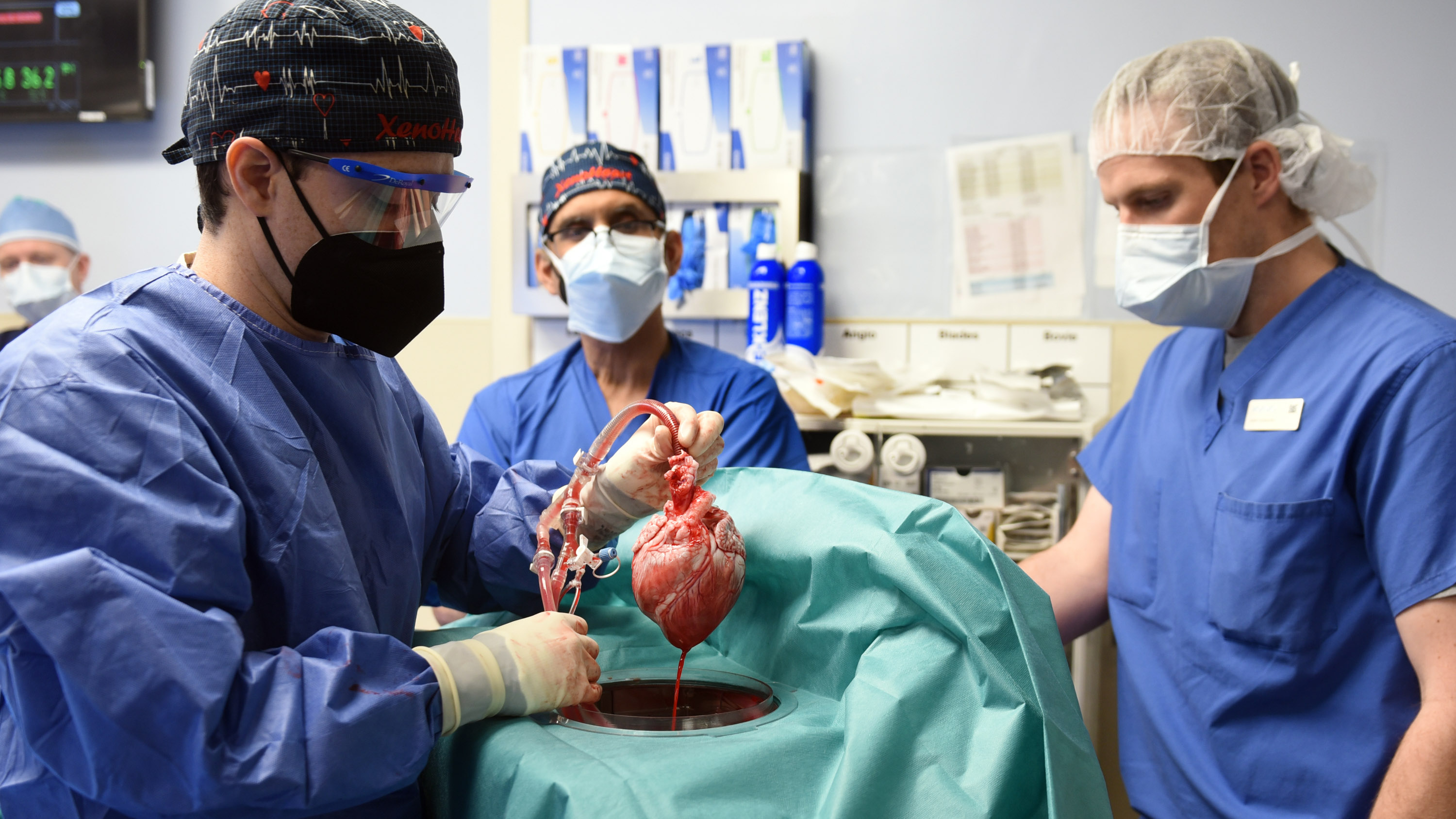
The pig heart transplanted into an American patient earlier this year in a landmark operation carried a porcine virus that may have derailed the experiment and contributed to his death two months later, say transplant specialists.
David Bennett Sr. was near death in January when he received a genetically edited pig heart in a pioneering between-species transplant that has been hailed as a success—and was, at first.
A few days after his heart was replaced with one from a pig, Bennett was sitting up in bed. His new heart was pumping fantastically and performing like a “rock star,” according to his transplant surgeon, Bartley Griffith of the University of Maryland School of Medicine.
But about 40 days later Bennett, who was 57, took a turn for the worse. After two months he was dead. In a statement released by the university in March, a spokesperson said there was “no obvious cause identified at the time of his death” and that a full report was pending.
Now MIT Technology Review has learned that Bennett’s heart was affected by porcine cytomegalovirus, a preventable infection that is linked to devastating effects on transplants.
The presence of the pig virus and the desperate efforts to defeat it were described by Griffith during a webinar streamed online by the American Society of Transplantation on April 20. The issue is now a subject of wide discussion among specialists, who think the infection was a potential contributor to Bennett’s death and a possible reason why the heart did not last longer.
“We are beginning to learn why he passed on,” said Griffith, who believes that the virus “maybe was the actor, or could be the actor, that set this whole thing off.”
The heart swap in Maryland was a major test of xenotransplantation, the process of moving tissues between species. But because the special pigs raised to provide organs are supposed to be virus-free, it now appears that the experiment was compromised by an unforced error. The biotechnology company that raised and engineered the pigs, Revivicor, declined to comment and has made no public statement about the virus.
“It was surprising. That pig is supposed to be clean of all pig pathogens, and this is a significant one,” says Mike Curtis, CEO of eGenesis, a competing company that is also breeding pigs for transplant organs. “Without the virus, would Mr. Bennett have lived? We don’t know, but the infection didn’t help. It likely contributed to the failure.”
10-gene pig
The detection of the pig virus in Bennett's heart is not necessarily all bad news for xenotransplantation. If a pig virus played a role, it could mean a virus-free heart xenotransplant could last much longer. Some surgeons think the latest gene-modified organs could in theory keep beating for years—and more rigorous procedures should be able to screen out the virus.
“If this was an infection, we can likely prevent it in the future,” Griffith said during his presentation.
The biggest obstacle to animal-organ transplants is the human immune system, which ferociously attacks foreign cells in a process called rejection. To avoid rejection, companies have been engineering pigs—removing some genes and adding others—to give their tissue a stealth profile that hides from immune attack.
The version used in Maryland came from a pig with 10 gene modifications developed by Revivicor, a subsidiary of United Therapeutics.
Following promising tests of such pig organs in baboons, three US transplant teams launched the first human studies starting in late 2021. Surgeons at New York University and the University of Alabama each attached pig kidneys to brain-dead people, but the University of Maryland went a step further when Griffith stitched a pig heart into Bennett’s chest in early January.
Transferring pig viruses to humans has been a worry—some fear xenotransplantation could set off a pandemic if a virus were to adapt inside a patient’s body and then spread to doctors and nurses. The concern could be serious enough to require lifelong monitoring for patients.
However, the specific type of virus found in Bennett’s donor heart is not believed capable of infecting human cells, says Jay Fishman, a specialist in transplant infections at Massachusetts General Hospital. Fishman thinks there is “no real risk to humans” of its spreading further.
Instead, the problem is that pig cytomegalovirus is linked to reactions that can damage the organ and the patient—with catastrophic results. Two years ago, for instance, German researchers reported that pig hearts transplanted into baboons lasted only a couple of weeks if the virus was present, while organs free from the infection could survive more than half a year.
Those researchers said they found “astonishingly high” virus levels in pig hearts removed from baboons. They think the virus could go haywire not just because the baboons’ immune systems were suppressed with drugs, but also because the pig immune system was no longer there to keep the virus in check. It “seems very likely the same may happen in humans,” they warned at the time.
Joachim Denner of the Institute of Virology at the Free University of Berlin, who led that study, says the solution to the problem is more accurate testing. The US team appears to have tested the pig’s snout for the virus, but often it is lurking deeper in the tissues.
“It’s a latent virus and hard to detect,” says Denner. “But if you test the animal better, it will not happen. The virus can be detected and easily removed from pig populations, but unfortunately they didn’t use a good assay and didn’t detect the virus, and this was the reason. The donor pig was infected, and the virus was transmitted by the transplant.”
Denner says he still thinks the experiment was a “great success.” For instance, the first human-to-human heart transplant, in 1967, lasted only 18 days and, two years later, one in Germany endured just 27 hours.
Denner says that Bennett’s death cannot be blamed on the virus alone. “This patient was very, very, very ill. Do not forget that,” he says. “Maybe the virus contributed, but it was not the sole reason.”
Cause of death?
Bennett’s cause of death matters, because if his heart failed as the result of immune rejection, researchers might need to return to the drawing board. Instead, it’s now expected that companies like United Therapeutics and eGenesis, or academics working with them, will launch clinical trials of their pig organs within a year or two.
Bennett was offered a pig heart after Griffith applied to the US Food and Drug Administration for special permission to try an animal organ in a one-off transplant. He was considered a good candidate for the daring attempt because he was nearing death from heart failure and was ineligible for a scarce human heart for transplant owing to a history of disregarding medical advice.
On December 31, 2021, the FDA sent Griffith an email saying he could proceed to treat Bennett for “irreversible heart failure” if the patient and ethics monitors agreed.
Bennett’s condition remained fragile all along. Still, after the operation, his new pig heart was squeezing powerfully and looking “super normal,” according to Griffith. Even a biopsy taken on day 34 showed no signs of the feared immune attack.
“It was quite amazing. You go talk to this gentleman and he’s got a pig heart. Literally, he has a pig heart,” Griffith said. The result was all but miraculous, yet Griffith acknowledged that the medical team was in anguish about whether they were doing the right thing.
In some ways, he says, they were like a “blind group of squirrels” scurrying to manage Bennett's unprecedented condition as the days ticked by.
To keep tabs on the health of the pig heart, Griffith said, the team was constantly checking their patient with an array of cutting-edge blood tests. They used a DNA sequencer to scan his blood for floating fragments of pig genes—any increase would be a sign that heart cells were dying. Another novel test, developed by a company called Karius, screened Bennett’s blood for traces of hundreds of bacteria and viruses.
It was that test, run on a blood draw taken from Bennett 20 days after his surgery, that first returned “a little blip” indicating the presence of porcine cytomegalovirus, according to Griffith. But the levels were so low that the team thought the result could be in error, Griffith said, especially since the pigs were supposedly guaranteed free of the germ.
The doctors faced another problem—the special blood test was taking about 10 days to carry out. So they couldn’t yet know that inside Bennett’s new heart, the pig virus was starting to multiply fast and setting off what Griffith now believes was a possible “cytokine explosion”—a storm of immune-system molecules.
Even without up-to-the-minute tests, a serious problem became apparent around day 43 of the experiment. That day, Bennett woke up warm to the touch and breathing hard. “He looked really funky. Something happened to him. He looked infected,” said Griffith. “He lost his attention and wouldn’t talk to us.”
The doctors then faced a common problem in transplant medicine: how to fight infections while still keeping the patient’s immune system in check. And they were also handicapped by what they didn’t know. Not only were they still guessing at the true extent of the infection, but no one had ever treated a human for this particular pig virus, according to Griffith's account.
They ended up giving Bennett a last-resort drug called cidofovir, sometimes used in AIDS patients. And since his immune system was so weak, they also gave him intravenous immunoglobulin—antibodies collected from blood donors.
Bennett looked better 24 hours later and was sitting up in a chair, “so we were all kind of relaxing—we dodged that bump,” says Griffith. But the relief didn’t last long. A week later Bennett’s again looked terrible and his heart started to fail.
Now Griffith wonders if Bennett was hit by the same syndrome previously seen in baboons that received infected pig hearts. Somehow, the virus sets off a wider inflammation response, causing swelling and other effects.
Mass General’s Fishman says from what he’s heard of Bennett’s case, “it does sound like the syndrome was driven by [the virus].”
Still, it’s too soon to say for sure why Bennett died and researchers are still sifting through complex and contradictory clues. The doctors also worry they made a mistake by giving him human antibodies—something they did twice. Later tests showed that those blood products had contained some anti-pig antibodies and they might have damaged the organ too.
Even so, Griffith said that a biopsy of Bennett’s pig heart late in the experiment did not show telltale signs it had been rejected by his immune system—which had been the biggest fear all along, and what the special gene-edited pigs were designed to avoid in the first place.
Instead, the pattern of damage—which was unusually “bland,” according to Griffith—was similar to that seen in the German baboons. During his presentation, Griffith painted a picture of how the viral syndrome might have caused the heart to fail—starting with that unexpected “blip” in a test result, building to a bigger infection, and then releasing a damaging cascade of inflammation.
“I personally suspect he developed a capillary leak in response to his inflammatory explosion, and that filled his heart with edema, the edema turned into fibrotic tissue, and he went into severe and unreversing diastolic heart failure,” said Griffith.
The researchers involved have said the procedure was worth it because of the “invaluable insights” they gained. In a statement released by the university in March, Bennett’s son shared similar sentiments. “We also hope that what was learned from his surgery will benefit future patients and hopefully one day, end the organ shortage that costs so many lives each year,” he said.
However, the presence of the virus—whose risks were already well documented—could now factor into some people’s questions over whether the experiment should have taken place at all. “It’s a big red flag,” says Arthur Caplan, a bioethicist at New York University. If doctors can’t prevent or control infection, “then such experiments are tough to justify.” Caplan says he has concerns about whether the risky procedure was appropriate, since Bennett was clinging to life and his ability to consent or end his participation isn't clear.
Bennett’s doctors have called him a bold volunteer who showed plenty of fight. “These losses are hard,” Griffith said during the webinar. “This was a patient. It wasn't an experiment to us. All he wanted to do was live. In fact, he was such a funny guy. On the way in to get his pig heart transplant, he looked at me and he said squarely, ‘Are you sure I can’t get a human heart?’”
Deep Dive
Biotechnology and health
The Biggest Questions: What is death?
New neuroscience is challenging our understanding of the dying process—bringing opportunities for the living.
I received the new gene-editing drug for sickle-cell disease. It changed my life.
As a patient enrolled in a clinical trial for Vertex’s new treatment, I was among the first to experience CRISPR’s transformative effects.
The lucky break behind the first CRISPR treatment
Gene editing for sickle-cell is here. This is how researchers knew what DNA to change.
Medical microrobots that can travel inside your body are (still) on their way
Microrobots released into the body could bust up clots, deliver cancer drugs, and even guide listless sperm to their target.
Stay connected
Get the latest updates from
MIT Technology Review
Discover special offers, top stories, upcoming events, and more.